実施日 : 2013年03月18日(月)
Notice: Medical Innovation Press Tour in Yokohama (March18, 2013)
投稿日 : 2013年08月22日
 On February 22, 2013, the government announced that it would establish an Office for Healthcare and Medical Strategy under Chief Cabinet Secretary Yoshihide Suga. This office is intended to enable the government, the industrial world and research institutions, in concert, to go about medical technology innovation such as putting regenerative medicine using iPS cells into practical use or developing new medicines for intractable illnesses.
On February 22, 2013, the government announced that it would establish an Office for Healthcare and Medical Strategy under Chief Cabinet Secretary Yoshihide Suga. This office is intended to enable the government, the industrial world and research institutions, in concert, to go about medical technology innovation such as putting regenerative medicine using iPS cells into practical use or developing new medicines for intractable illnesses.
Japanese medicine has been, so to speak, "good at technology but poor in business" in the world. Japan has a high level of basic research and superior technology, but too-strict regulations, vertically integrated administration, and the gap between researchers and drug manufacturers are, in many cases, preventing brilliant R&D results from being put into practical use (so-called “Death Valley” problem). As a result, Japan’s imports of medicines and medical equipment greatly exceed exports in trade amount.
Trying to change the situation and raise the international competitiveness of the medical industry, Japan is now veering toward“medical innovation.” As the growth strategy, one of the three arrows in Abenomics, puts priority on “medical services,”it is expected that R&D in this field will further advance in the future by deregulation and industry-government-academia collaboration. For R&D on “regenerative medicine,” in particular, whose share of the domestic market, the Ministry of Economy, Trade and Industry estimates, will reach 1.6 trillion yen in 2030, the government is ready to give strong support in terms of legislation and financial assistance.
 Some areas are making good use of the government’s special zone system for full-blown R&D in the most advanced medicine. A coastal area straddling Yokohama City and Kawasaki City was designated as the Keihin Coastal Area Life Innovation Comprehensive Special Zone for International Competitiveness toward the end of 2011. At the Advanced Medical Research Center of Yokohama City University, the core medical research organization in Yokohama City, researchers, in cooperation with private companies, are trying to realize state-of-the-art medical treatment and develop innovative medical drugs and equipment.
Some areas are making good use of the government’s special zone system for full-blown R&D in the most advanced medicine. A coastal area straddling Yokohama City and Kawasaki City was designated as the Keihin Coastal Area Life Innovation Comprehensive Special Zone for International Competitiveness toward the end of 2011. At the Advanced Medical Research Center of Yokohama City University, the core medical research organization in Yokohama City, researchers, in cooperation with private companies, are trying to realize state-of-the-art medical treatment and develop innovative medical drugs and equipment.
This tour covers Japan’s innovative medical science, including a briefing by Cabinet Councilor Hideaki Nakagaki, who serves as Deputy Director of the newly-established Office for Healthcare and Medical Strategy, on its direction for medical innovation; a briefing by Yokohama City on the Special Zone; and a visit to a new research building of the Advanced Medical Research Center to be opened this April.
* The tour is hosted by Yokohama City with FPCJ’s cooperation in planning and operation.
<Presentations>
1. Briefing by Office for Healthcare and Medical Strategy
Briefer: Deputy Director Hideaki Nakagaki (Cabinet Councilor)
-Medical innovation as a national strategy-
The Office has been newly established to strengthen the government’s leadership in boosting Japan’s international competitiveness in the medical industry. Special Adviser to Prime Minister Hiroto Izumi became the head of the Office, while Chief Cabinet Secretary Yoshihide Suga serves as the supervisor. Suga said that under the Office’s leadership, the government aims to “develop the medical sector, pharmaceutical products and medical devices and equipment as strategic industries that will form a key pillar for Japan's economic revitalization.”
〇On the tour, Cabinet Councilor Hideaki Nakagaki, who serves as Deputy Director of the Office, will talk about how the new organization plans to advance the country’s medical innovation and how it plans to make the innovation lead to economic revitalization.
2. Brifeing by Yokohama City
-Keihin Coastal Area Life Innovation Comprehensive Special Zone for International Competitiveness-
 The Keihin Coastal Area straddling Yokohama City and Kawasaki City is home to many life-science-related research centers and enterprises such as the medical department of Yokohama City University and RIKEN. Utilizing this local advantage, this Zone aims to create a base for strengthening Japan’s international competitiveness in pharmaceutical products, medical devices and related industries. The Zone covers the four districts of Suehiro (Tsurumi Ward), Fukuura (Kanazawa Ward) and Minatomirai in Yokohama City and Tomomachi in Kawasaki City. In these areas, researchers can tackle pioneering research and development thanks to relaxed regulations and tax breaks.
The Keihin Coastal Area straddling Yokohama City and Kawasaki City is home to many life-science-related research centers and enterprises such as the medical department of Yokohama City University and RIKEN. Utilizing this local advantage, this Zone aims to create a base for strengthening Japan’s international competitiveness in pharmaceutical products, medical devices and related industries. The Zone covers the four districts of Suehiro (Tsurumi Ward), Fukuura (Kanazawa Ward) and Minatomirai in Yokohama City and Tomomachi in Kawasaki City. In these areas, researchers can tackle pioneering research and development thanks to relaxed regulations and tax breaks.
〇On the tour, a Yokohama City official will brief on the outline of the Special Zone.
3. The Advanced Medical Research Center (AMRC) of Yokohama City University (YCU)
http://www.yokohama-cu.ac.jp/amedrc/eng/ (1) Briefing by Dr. Hisashi Hirano, Director
(1) Briefing by Dr. Hisashi Hirano, Director
AMRC was established in October 2006 to be a bridge over the “Death Valley” connecting the achievements of basic research with clinical treatment. At the new research building to be opened in April 2013, cutting-edge R&D will be conducted for putting iPS cells into practical use and also for cancer prevention, diagnosis and treatment. A university-industry collaboration laboratory in the new building will house Shiseido and JTEC Corporation, a bio venture firm; it is expected that university-industry joint research in regenerative medicine will be conducted in a field very close to practical use.
〇On the tour, Dr. Hisashi Hirano, AMRC Director, will give an outline of AMRC, following the introduction of YCU and its efforts to internationalize the university.
(2) Laparoscopic Surgery Simulator (Lap-Pass)
-Virtual surgery made possible using patient data-
 Surgeons need experience to brush up their surgical skills, but patients, on the other hand, want an operation by a seasoned doctor with a lot of training and experience. This surgical operation simulator can contribute to overcoming this problem in clinical doctors. Based on the data obtained by CT or MRI, this simulator provides the affected part of a patient in three-dimensional CG images so that the doctor can perform simulated laparoscopic surgery training. The positions of internal organs, which differ slightly from person to person, or even a sense of surgical tools touching an organ, can be reproduced. YMC, Mitsubishi Precision, a manufacturer of precision equipment, and a software company have been jointly working on its research and development; when put into practical use, the simulator is expected to improve medical safety.
Surgeons need experience to brush up their surgical skills, but patients, on the other hand, want an operation by a seasoned doctor with a lot of training and experience. This surgical operation simulator can contribute to overcoming this problem in clinical doctors. Based on the data obtained by CT or MRI, this simulator provides the affected part of a patient in three-dimensional CG images so that the doctor can perform simulated laparoscopic surgery training. The positions of internal organs, which differ slightly from person to person, or even a sense of surgical tools touching an organ, can be reproduced. YMC, Mitsubishi Precision, a manufacturer of precision equipment, and a software company have been jointly working on its research and development; when put into practical use, the simulator is expected to improve medical safety.
*Laparoscopic Surgery: a surgical operation performed by doctors who, while operating, watch the affected part through a laparoscope put into a small incision on the patient’s abdomen. Compared with conventional laparotomy, this surgery is less stressful to the patient but requires high surgical skills of the doctor.
〇On the tour, a doctor or a researcher will brief on the outline, development history and significance of the surgical simulator, and then participants will experience mock surgery using this simulator.
(3) Home Ultrasonograph
-Lymphedema and breast cancer can be checked at home!-
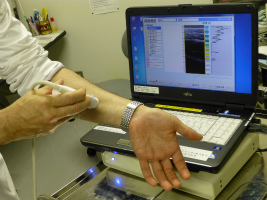 It is said that one out of two breast cancer patients develops lymphedema, a symptom of swollen limbs that appears when tissue fluid that cannot go back to the lymph vessels after a patient has had her lymph nodes removed in an operation. In order to detect this disease at an early stage or to help assess treatment effects, YCU, a venture firm Global Health Co. Ltd. and other companies have been jointly developing an ultrasonograph miniaturized for home use. When it is connected to a PC and its sensor is pressed on the skin with a special gel applied, this equipment produces on the PC ultrasonic images of accumulated lymphatic fluid. Yokohama City University Hospital has just begun free lending of a few units to some patients for clinical testing. The existing Pharmaceutical Affairs Act limits the use of this equipment to medical personnel, and home use is not allowed; the lending in Yokohama is possible only because the city can take advantage of deregulation in a special zone. They are now planning to develop a device that makes it possible to check breast cancer at home.
It is said that one out of two breast cancer patients develops lymphedema, a symptom of swollen limbs that appears when tissue fluid that cannot go back to the lymph vessels after a patient has had her lymph nodes removed in an operation. In order to detect this disease at an early stage or to help assess treatment effects, YCU, a venture firm Global Health Co. Ltd. and other companies have been jointly developing an ultrasonograph miniaturized for home use. When it is connected to a PC and its sensor is pressed on the skin with a special gel applied, this equipment produces on the PC ultrasonic images of accumulated lymphatic fluid. Yokohama City University Hospital has just begun free lending of a few units to some patients for clinical testing. The existing Pharmaceutical Affairs Act limits the use of this equipment to medical personnel, and home use is not allowed; the lending in Yokohama is possible only because the city can take advantage of deregulation in a special zone. They are now planning to develop a device that makes it possible to check breast cancer at home.
〇On the tour, Professor Jiro Maekawa, who specializes in plastic surgery and engages in developing the ultrasonography, will brief on the outline, development history and significance of the device. Participants will also experience checking by the ultrasonography.
(4) Cellome Analyses Division
-Utilizing iPS cells for future regeneration medicine and cancer treatment- This laboratory conducts analyses at the cellular level, aiming to develop innovative treatments under the general framework of "gene therapy and regenerative medicine" for cancer, lifestyle-related diseases and infectious diseases. In June 2012, a research team led by Professor Hideki Taniguchi succeeded in creating a tiny human liver from iPS cells transplanted into the body of a mouse. In January 2013, a research team led by Professor Akihide Ryo succeeded in creating cancer stem cells from iPS cells. Scientists believe that cancer spreads to other organs or recurs due to cancer stem cells, which continue to produce cancer cells without end. Prof. Ryo’s achievement is expected to help develop medicine to attack the stem cells, enabling researchers to artificially create and culture them.
This laboratory conducts analyses at the cellular level, aiming to develop innovative treatments under the general framework of "gene therapy and regenerative medicine" for cancer, lifestyle-related diseases and infectious diseases. In June 2012, a research team led by Professor Hideki Taniguchi succeeded in creating a tiny human liver from iPS cells transplanted into the body of a mouse. In January 2013, a research team led by Professor Akihide Ryo succeeded in creating cancer stem cells from iPS cells. Scientists believe that cancer spreads to other organs or recurs due to cancer stem cells, which continue to produce cancer cells without end. Prof. Ryo’s achievement is expected to help develop medicine to attack the stem cells, enabling researchers to artificially create and culture them.
〇On the tour, researchers in the Division will show you the laboratory and talk about their research (outline, meaning, achievements, prospects, etc.) Through a microscope or on a PC screen, you will observe iPS cells, a human liver and cancer stem cells created by iPS cells.
5) Proteome Analyses Division
-Paving the way for early cancer detection and custom-made treatment-
 This laboratory conducts analyses on protein. Cancer is not a single disease. For example, there are several different types of ovarian cancer, and treatment varies with each kind. Therefore, to provide suitable treatment for each patient, doctors need to make an exact diagnosis on the type of cancer by identifying “abnormal proteins” that constitute cancer cells. But since there are more than 100,000 types of protein in a human body, it used to take about 2 years to identify the type of a particular protein. This problem was solved with the technique of mass spectrometry, which drew public attention when engineer Koichi Tanaka of Shimazu Corporation won a Nobel Prize in chemistry in 2002. The mass of a protein is determined by the type of the protein; and thus if one knows the mass of a protein, then he can tell the type of the protein. More advanced mass spectrometers have been developed these days, and with YCU’s latest spectrometers, it takes only a couple of days to identify the type of a protein. Nowadays, researchers can diagnose illnesses from a single drop of blood.
This laboratory conducts analyses on protein. Cancer is not a single disease. For example, there are several different types of ovarian cancer, and treatment varies with each kind. Therefore, to provide suitable treatment for each patient, doctors need to make an exact diagnosis on the type of cancer by identifying “abnormal proteins” that constitute cancer cells. But since there are more than 100,000 types of protein in a human body, it used to take about 2 years to identify the type of a particular protein. This problem was solved with the technique of mass spectrometry, which drew public attention when engineer Koichi Tanaka of Shimazu Corporation won a Nobel Prize in chemistry in 2002. The mass of a protein is determined by the type of the protein; and thus if one knows the mass of a protein, then he can tell the type of the protein. More advanced mass spectrometers have been developed these days, and with YCU’s latest spectrometers, it takes only a couple of days to identify the type of a protein. Nowadays, researchers can diagnose illnesses from a single drop of blood.
〇On the tour, Professor Hisashi Hirano, head of the Division, will show you the laboratory and talk about their research (outline, meaning, achievements, prospects, etc.)
【Tour itinerary and application details】
1. Itinerary (Tentative): Monday, March 18, 2013
10:00-11:00 Briefing by Office for Healthcare and Medical Strategy
11:00-11:30 Briefing by Yokohama City on Keihin Coastal Area Life Innovation Comprehensive Special Zone for International Competitiveness
11:30-12:15 Lunch
12:15-13:15 Move by chartered bus
13:15 Arrive at Advanced Medical Research Center (AMRC) of Yokohama City University (YCU)
13:30-13:45 Briefing on outline of Yokohama City University
13:40-14:20 Briefing on outline of AMRC by Professor Hisashi HIRANO, head of AMRC
14:20-14:50 Proteome Analyses Division 14:50-15:20 Cellome Analyses Division
15:20-15:50 Laparoscopic Surgery Simulator (Lap-Pass)
15:50-16:20 Home Ultrasonograph
(Wrap up and disperse)
2. Qualification: Bearer of Gaimusho Press Registration Card
3. Cost: 1000-1500 yen for lunch (The money will be collected on the tour.)
4. Participants: Limited to the first 10 applicants
(Only one reporter and one photographer from each company, but two participants from each TV team will be acceptable.)
If the number of applicants exceeds 10, an upper limit may be set on the number of participants from each country.
5. FPCJ Contact: Ms. Ishikawa (Tel: 03-3501-3405)
6. Remarks:
(1) There may be some restrictions on photographing and filming at the tour sites. Please follow the instructions of the officials on duty.
(2) FPCJ and the City of Yokohama will not be liable for any inconvenience, trouble or accident that might occur in the course of the tour.


